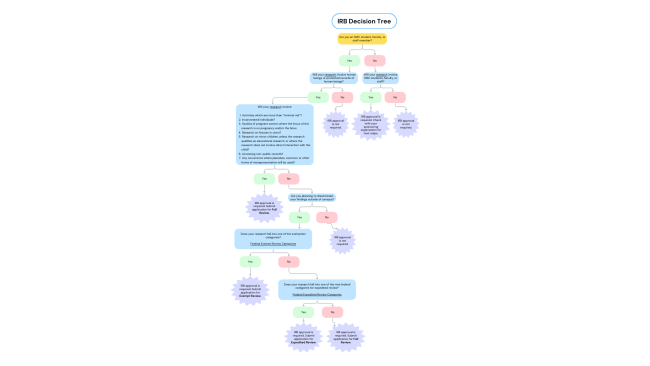
Types of Review
All human subject research that does not qualify for Exempt Review or Expedited Review will undergo Regular (Full Committee) Review. Typically, research requiring Regular Review exceeds the standard for “minimal risk” and/or involves vulnerable populations.
The following five categories of research always require Regular (Full) Review:
- research involving prisoners
- studies of pregnant women where the focus of the research is on pregnancy and/or the fetus
- research on fetuses in utero
- research on minor children unless the research qualifies as educational research in the sense of IV.B.1a. or IV.B.1b above, or where the research does not involve direct interaction with the child
- research using non-public records.
Applications requiring Regular Review will be reviewed by the full committee. Thus, researchers need to allow enough time for a full committee review of the application.
Your complete application must include the following components:
- CITI training (Social and Behavioral Research Module, Basic Course/Refresher) completion certificate for all researchers listed in the application, including the faculty supervising the student research.
- Application Form (Save the file as “Your Name_Application” in one file.)
- Appendices (Appendices should be saved as “Your Name_Appendices” in one file. We do not accept a zip file).
If your study does not involve vulnerable populations, involves no more than minimal risk, and meets one or more of the categories listed below, it can be reviewed as an exempt study. Exempt studies will be reviewed by the Chair or a designated IRB member.
Be sure to mark which exemption category your study falls under on the application form.
Based on 45 CFR 46.401(b), the below exemption category applies to research with children as follows:
- The use of educational tests is exempt, but survey or interview procedures are not exempt
- Observations of public behavior is exempt only when the investigator does not participate in the observed activities
Category 1
Research conducted in established or commonly accepted educational settings, involving normal educational practices, such as:
(i) research on regular and special education instructional strategies; OR
(ii) research on the effectiveness of or the comparison among instructional techniques, curricula, or classroom management methods.
Category 2
Research involving the use of educational tests (cognitive, diagnostic, aptitude, achievement), survey procedures, interview procedures, observation of public behavior, IF:
(i) the information obtained is recorded in such a manner that subjects CANNOT be identified, directly or through identifiers linked to the subjects; OR
(ii) any disclosure of the subject's responses outside of the research could NOT reasonably place the subject at risk of criminal or civil liability or be damaging to the subject's financial standing, employability, or reputation.
*This exemption does not apply to children except for research involving observation of public behavior when the investigator does not interact with the children. Workplace meetings and activities, as well as classroom activities, are not considered "public behavior".
Category 3
Research involving the use of educational tests (cognitive, diagnostic, aptitude, achievement), survey procedures, or observation of public behavior that is not exempt under Category 2, IF:
(i) the subjects are elected or appointed public officials or candidates for public office; OR
(ii) federal statute requires confidentiality of identifiable information to be maintained permanently. *In most cases, managers and staff in public agencies are not "public officials".
Category 4 (Fill out a Secondary Data Analysis Form instead of this Non-Exempt/Exempt Form)
Research involving collection or study of existing data, documents, records, or specimens, IF:
(i) these sources are publicly available; OR
(ii) the information is recorded by the researcher in such a manner that subjects cannot be identified, directly or through identifiers linked to the subjects.
Category 5
Research and demonstration projects which are conducted by or subject to the approval of Department or Agency heads, and which are designed to study, evaluate, or otherwise examine:
(i) public benefit or service programs;
(ii) procedures for obtaining benefits or services under those programs; OR
(iii) possible changes in or alternatives to those programs; OR
(iv) changes in methods of payment for benefits under those programs.
Category 6
Taste and food quality evaluation and consumer acceptance studies, IF: (i) wholesome foods without additives are consumed, OR (ii) a food is consumed that contains a food ingredient at or below the level and for a use found to be safe by the Food and Drug Administration (FDA) or approved by the Environmental Protection Agency (EPA) or the Food Safety and Inspection Service (FSIS) of the US Department of Agriculture (USDA); OR (iii) a food is consumed that contains an agricultural chemical or environmental contaminant at or below the level found to be safe by the FDA or approved by the EPA or the FSIS of the USDA.
Expedited review may be used for minor changes to previously approved Saint Mary’s College proposals and for new projects which involve no more than minimal risk and only involve human subjects in one or more of the categories listed below. Proposals submitted for expedited review will be reviewed by the IRB Chair or a Designated Representative of the Committee.
- Researchers are asked to indicate on the application form the category, listed below, upon which the expedited review request is based.
Applicability
- Research activities that (1) present no more than minimal risk to human subjects, and (2) involve only procedures listed in one or more of the following categories, may be reviewed by the IRB through the expedited review procedure authorized by 45CFR46.110 and 21 CFR 56.110. The activities listed should not be deemed to be of minimal risk simply because they are included on this list. Inclusion on this list merely means that the activity is eligible for review through the expedited review procedure when the specific circumstances of the proposed research involve no more than minimal risk to human subjects.
- The categories in this list apply regardless of the age of subjects, except as noted.
- The expedited review procedure may not be used where identification of the subjects and/or their responses would reasonably place them at risk of criminal or civil liability or be damaging to the subjects financial standing, employability, insurability, reputation, or be stigmatizing, unless reasonable and appropriate protections will be implemented so that risks related to invasion of privacy and breach of confidentiality are no greater than minimal.
- The expedited review procedure may not be used for classified research involving human subjects.
- IRBs are reminded that the standard requirements for informed consent (or its waiver, alteration, or exception) apply regardless of the type of review--expedited or convened--utilized by the IRB.
- Categories one (1) through seven (7) pertain to both initial and continuing IRB review.
Research Categories
- Clinical studies of drugs and medical devices only when condition (a) or (b) is met.
- Research on drugs for which an investigational new drug application (21 CFR Part 312) is not required. (Note: Research on marketed drugs that significantly increases the risks or decreases the acceptability of the risks associated with the use of the product is not eligible for expedited review.)
- Research on medical devices for which (i) an investigational device exemption application (21 CFR Part 812) is not required; or (ii) the medical device is cleared/approved for marketing and the medical device is being used in accordance with its cleared/approved labeling.
- Research on drugs for which an investigational new drug application (21 CFR Part 312) is not required. (Note: Research on marketed drugs that significantly increases the risks or decreases the acceptability of the risks associated with the use of the product is not eligible for expedited review.)
- Collection of blood samples by finger stick, heel stick, ear stick, or venipuncture as follows:
- from healthy, nonpregnant adults who weigh at least 110 pounds. For these subjects, the amounts drawn may not exceed 550 ml in an 8 week period and collection may not occur more frequently than 2 times per week; or
- from other adults and children 2, considering the age, weight, and health of the subjects, the collection procedure, the amount of blood to be collected, and the frequency with which it will be collected. For these subjects, the amount drawn may not exceed the lesser of 50 ml or 3 ml per kg in an 8 week period and collection may not occur more frequently than 2 times per week.
- from healthy, nonpregnant adults who weigh at least 110 pounds. For these subjects, the amounts drawn may not exceed 550 ml in an 8 week period and collection may not occur more frequently than 2 times per week; or
- Prospective collection of biological specimens for research purposes by noninvasive means.
Examples: (a) hair and nail clippings in a nondisfiguring manner; (b) deciduous teeth at time of exfoliation or if routine patient care indicates a need for extraction; © permanent teeth if routine patient care indicates a need for extraction; (d) excreta and external secretions (including sweat); (e) uncannulated saliva collected either in an unstimulated fashion or stimulated by chewing gumbase or wax or by applying a dilute citric solution to the tongue; (f) placenta removed at delivery; (g) amniotic fluid obtained at the time of rupture of the membrane prior to or during labor; (h) supra- and subgingival dental plaque and calculus, provided the collection procedure is not more invasive than routine prophylactic scaling of the teeth and the process is accomplished in accordance with accepted prophylactic techniques; (i) mucosal and skin cells collected by buccal scraping or swab, skin swab, or mouth washings; (j) sputum collected after saline mist nebulization.
- Collection of data through noninvasive procedures (not involving general anesthesia or sedation) routinely employed in clinical practice, excluding procedures involving x-rays or microwaves. Where medical devices are employed, they must be cleared/approved for marketing. (Studies intended to evaluate the safety and effectiveness of the medical device are not generally eligible for expedited review, including studies of cleared medical devices for new indications.)
Examples: (a) physical sensors that are applied either to the surface of the body or at a distance and do not involve input of significant amounts of energy into the subject or an invasion of the subject=s privacy; (b) weighing or testing sensory acuity; (c) magnetic resonance imaging; (d) electrocardiography, electroencephalography, thermography, detection of naturally occurring radioactivity, electroretinography, ultrasound, diagnostic infrared imaging, doppler blood flow, and echocardiography; (e) moderate exercise, muscular strength testing, body composition assessment, and flexibility testing where appropriate given the age, weight, and health of the individual.
- Research involving materials (data, documents, records, or specimens) that have been collected, or will be collected solely for nonresearch purposes (such as medical treatment or diagnosis). (Note: Some research in this category may be exempt from the HHS regulations for the protection of human subjects. 45 CFR 46.101(b)(4). This listing refers only to research that is not exempt.)
- Collection of data from voice, video, digital, or image recordings made for research purposes.
- Research on individual or group characteristics or behavior (including, but not limited to, research on perception, cognition, motivation, identity, language, communication, cultural beliefs or practices, and social behavior) or research employing survey, interview, oral history, focus group, program evaluation, human factors evaluation, or quality assurance methodologies. (Note: Some research in this category may be exempt from the HHS regulations for the protection of human subjects. 45 CFR 46.101(b)(2) and (b)(3). This listing refers only to research that is not exempt.)
- Continuing review of research previously approved by the convened IRB as follows:
- Where (i) the research is permanently closed to the enrollment of new subjects; (ii) all subjects have completed all research-related interventions; and (iii) the research remains active only for long-term follow-up of subjects; or
- where no subjects have been enrolled and no additional risks have been identified; or
- where the remaining research activities are limited to data analysis.
- Where (i) the research is permanently closed to the enrollment of new subjects; (ii) all subjects have completed all research-related interventions; and (iii) the research remains active only for long-term follow-up of subjects; or
- Continuing review of research, not conducted under an investigational new drug application or investigational device exemption where categories two (2) through eight (8) do not apply but the IRB has determined and documented at a convened meeting that the research involves no greater than minimal risk and no additional risks have been identified.
Policy
Research activities within a course may be shared with the campus community without the IRB review if ALL of the following Course Exemption Criteria are met.
- The data or the results of analysis are NOT disseminated outside of the campus. For example, publishing in ProQuest is considered outside of campus.
- The study is conducted anonymously, and the reporting of the data maintains the confidentiality of direct and indirect personal identifiers (i.e., anonymity in reporting the data).
- The study does not involve children, prisoners, or institutionalized persons.
- The study does not purposively collect data from the following vulnerable populations:
- Pregnant women
- Individuals with terminal illness, cognitive impairments, or mental or physical disabilities
- The data collection site does not require IRB approval.
- The study does not trigger emotional or physical distress (see IRB application form for the complete list of examples), such as
- Any probing for personal or sensitive information in surveys or interviews that might be experienced as an invasion of privacy (such as self-disclosure of personal information)
- Presenting materials to participants that they might consider sensitive, offensive, threatening, or degrading
- Distress resulting from the topic of the study (such as surviving political violence or natural disaster)
- Deception (lying to the participants—includes both withholding and misinforming)
Faculty Responsibility
Instructors who wish to provide students with the opportunity to share the results of the data gathering activities within courses with the campus community are required to meet the following requirements:
- Complete the CITI training (Social and Behavioral Research Module, Basic Refresher or other module appropriate for the type of study in the field).
- Stay informed about the ethical standards and regulatory requirements governing research activities with human participants.
- Make adequate time to consult with the students on their research activities to ensure compliance with the internal IRB policies, regulations and state law.
- Notify the IRB of unanticipated inadvertent or adverse effects within 10 days of learning them.
SMC IRB’s policy on research that examines teacher practice and pedagogy models and adopts the guidelines at Columbia University Teachers College.
It is important to remember that asking your own students to participate in your research is inherently coercive because they likely feel compelled to participate or perceive some intangible benefit to participation. Therefore, the IRB generally advises against approaching your own students to ask for their participation in your research unless the research is about your own teaching practice or pedagogy informed by the student learning of the course material.
There are some commonly experienced difficulties in preparing an IRB protocol for this type of research. Your rights and responsibilities as a classroom teacher are much broader than those of a researcher. As a teacher, you may be free to assign homework and activities, try a pedagogical intervention, observe and record classroom dynamic and discussion, etc. As a researcher, collection and recording of student activities for your research need to follow the design and procedures approved by the IRB. While research on teaching practice and pedagogy may appear to be self-study and in some ways auto-ethnographic, this type of research typically draws a conclusion about pedagogical efficacy and curriculum effectiveness upon student work as evidence of learning. When evidence and data are taken from human participants to make a claim about teaching practice patterns or curriculum structures that demonstrate effectiveness, your research becomes the IRB’s concern.
If you are conducting research that involves your own student’s coursework, your research project may be approved under exemption category #4 (Secondary Analysis of Data). Category 4 exemption may apply to studies that meet the following conditions:
- The course work (e.g., assignments, exams, quizzes) or activity observation (e.g., class discussion) is one that I am free to assign in my role as the instructor of record;
- The entire class can engage in the course work and activity, even if I only want to analyze data from a smaller subset of my students;
- The course work or activity does not require participation outside the classroom or outside normal class hours (except for homework assignments);
- The research does not involve a survey, interview, or focus group to obtain student personal account on teaching practice or pedagogy and/or topics outside of the teaching practice;
- Data can be de‐identified in any report or publication.
For the research that meets the above criteria for Category 4 exemption, you do not need to obtain child assent/consent or parent/guardian permission forms, but you must send an information letter to parents of minors and include the letter in the appendix to the IRB application material.
You may be required to complete an IRB review process for the school district or educational organization where your research will be conducted. If this is the case, you will need to obtain the approval from that process before you begin your research.
If your research involves interviewing or surveying your own students who are minors (e.g., K-12 in-service teachers), it needs to be reviewed as a non-exempt study.
If your research involves interviewing or surveying your own adult students (e.g., faculty in college classrooms), it needs to be reviewed as Category 1 exemption.
The Code of Federal Regulations (CFR) defines scholarly and journalistic activities (e.g., oral history, journalism, biography, literary criticism, legal research, and historical scholarship) as the collection and use of information that focus directly on the specific individuals about whom the information is collected. However, IRB at different institutions apply their own protocols to research activities in these fields in order to protect participants/interviewees.
- If you are interviewing people with cognitive disabilities or mental illness that make their decision to participate in the interview or to understand their rights as participants difficult, your study must be reviewed by the IRB.
- If you are interviewing children, it is very unlikely your study is oral history.
- If you are putting participants in potential psychological, physical, or legal risks, you should consult the IRB member.
The Oral History Association provides the principles and best practices for oral history. A few things to consider include:
- Interviewees' rights if and when they feel uncomfortable answering your questions or feel unsafe by disclosing some information during the interviews
- Agreement for any type of recording and treatment of the recorded material
- Clarity regarding how much of the personal identification the interviewees are willing to share
- Researchers' safety, liability, and responsibility
The most distinctive difference is whether the researcher is trying to illuminate a particular past through narratives (oral history) or to generalize the findings to the population (social science). The table below provides some examples for this distinction.
Oral History | Social Science |
| Interviewing Iraqi war veterans in order to chronicle/document their varied experiences | Interviewing veterans to determine the factors that led to and might be predictive of PTSD |
| Interviewing members of an SEIU union local in order to document their experience of a particular strike or organizing drive | Interviewing the union members in order to reveal the inherent contradictions of capitalism or the inevitablestruggle between labor and capital |
| Interviewing Black Lives Matter activists about the specific concerns that led to their involvement | Interviewing activists in order to draw conclusions about the motivations of social justice advocates across time, places, and movements |
If you are unsure whether your project is oral history or social science, please ask our oral history expert consultant, Dr. Gretchen Lamke-Santangelo (History, SOLA).